Cell and Gene Therapies: The Global Race to Cure Everything—Pioneering the Next Frontier in Medicine!
November 19, 2024 | Tuesday | Analysis
With 32.5% growth in clinical trials and breakthroughs in CAR-T therapies, AI, and manufacturing, the Asia-Pacific region emerges as a global hub, accelerating timelines and redefining patient care in the evolving CGT sector.
Cell and gene therapy (CGT) is at the frontier of medical innovation, promising transformative solutions for some of the most challenging diseases. According to Novotech’s whitepaper, "Cell and Gene Therapies – Global Clinical Trial Landscape (2024)," the sector is experiencing unparalleled growth, fueled by breakthroughs in research, supportive regulatory environments, and global collaboration. Below, we delve into the key insights shaping the CGT landscape and what they mean for the future of healthcare.
Unprecedented Growth in Clinical Trials
From 2019 to 2023, the global clinical trial landscape for cell and gene therapies grew by an impressive 32.5%, with over 1,500 drug candidates under development spanning phases 0 to III. The Asia-Pacific region now leads in trial activity, accounting for 44% of all trials. China, in particular, drives this growth due to favorable regulations, cost efficiencies, and robust infrastructure, followed closely by the United States, which holds 85% of North American trials.
Key Drivers:
- Hematological Focus: CAR-T therapies dominate, representing 32% of the pipeline, primarily targeting blood cancers such as diffuse large B-cell lymphoma (DLBCL).
- Emerging Targets: Increasing attention is directed toward solid tumors, with gastrointestinal and lung cancers being areas of heightened focus.
Innovative Therapies: Beyond Traditional Approaches
The scope of CGT is expanding to include novel technologies and targets. Beyond hematologic malignancies, gene therapies are being explored for rare genetic disorders like Duchenne Muscular Dystrophy and Sickle Cell Disease. Emerging approaches such as CAR-NK (natural killer) therapies offer reduced toxicity and hold potential for overcoming the immunosuppressive tumor microenvironment, particularly in solid tumors.
Technological Enablers:
- AI in Development: Artificial intelligence enhances target identification, predicts genetic modifications' outcomes, and streamlines clinical workflows, accelerating therapy development.
- CDMOs Transforming Manufacturing: Contract Development and Manufacturing Organizations (CDMOs) are crucial in addressing the complexity of CGT production, ensuring scalability while maintaining quality.
The Competitive Geography of CGT
While China leads in trial numbers, the United States remains a dominant force in CGT innovation, particularly in market adoption and cutting-edge R&D. Europe also plays a crucial role, with key contributions from countries like the UK and Germany, which are pivotal in regulatory harmonization and collaborative clinical studies. However, the Asia-Pacific region outshines others in patient recruitment efficiency, averaging 16.07 months compared to 21.15 months in the U.S. and 18.5 months in Europe. This advantage underscores the region’s capability in executing trials at a faster pace.
Recruitment and Scalability
Faster recruitment timelines in Asia-Pacific are attributed to several factors:
- Streamlined Processes: Simplified regulatory approvals and centralized ethics committees enable quicker trial initiation.
- Higher Population Density: A large patient pool ensures quicker enrollment, particularly for rare diseases.
- Cost Efficiency: Lower operational costs for site management and data collection make the region a favorable destination for trials.
Clinical Trial Activity and Recruitment Efficiency by Region
| Region | Clinical Trials (%) | Recruitment Timelines (Months) |
|---|---|---|
| China | 56 | 15.8 |
| United States | 85 | 21.15 |
| Europe (UK, Germany) | 20 | 18.5 |
| Asia-Pacific (Overall) | 44 | 16.07 |
Key Observations
- China: Driving 56% of Asia-Pacific’s trial activities, China’s role is critical in global CGT growth. Favorable policies such as government-funded research initiatives and accelerated approval pathways are key contributors.
- United States: Despite longer recruitment timelines, the U.S. remains a leader in innovation, conducting 85% of CGT trials in North America and benefiting from a robust network of academic and private research institutions.
- Europe: With a focus on regulatory harmonization, Europe offers a balanced ecosystem for trials, although recruitment timelines are slower than Asia-Pacific.
- Asia-Pacific: The region’s overall contribution of 44% to global trial activity highlights its growing prominence, bolstered by infrastructure investments and collaborations with international sponsors.
The following chart visually compares recruitment timelines across regions:
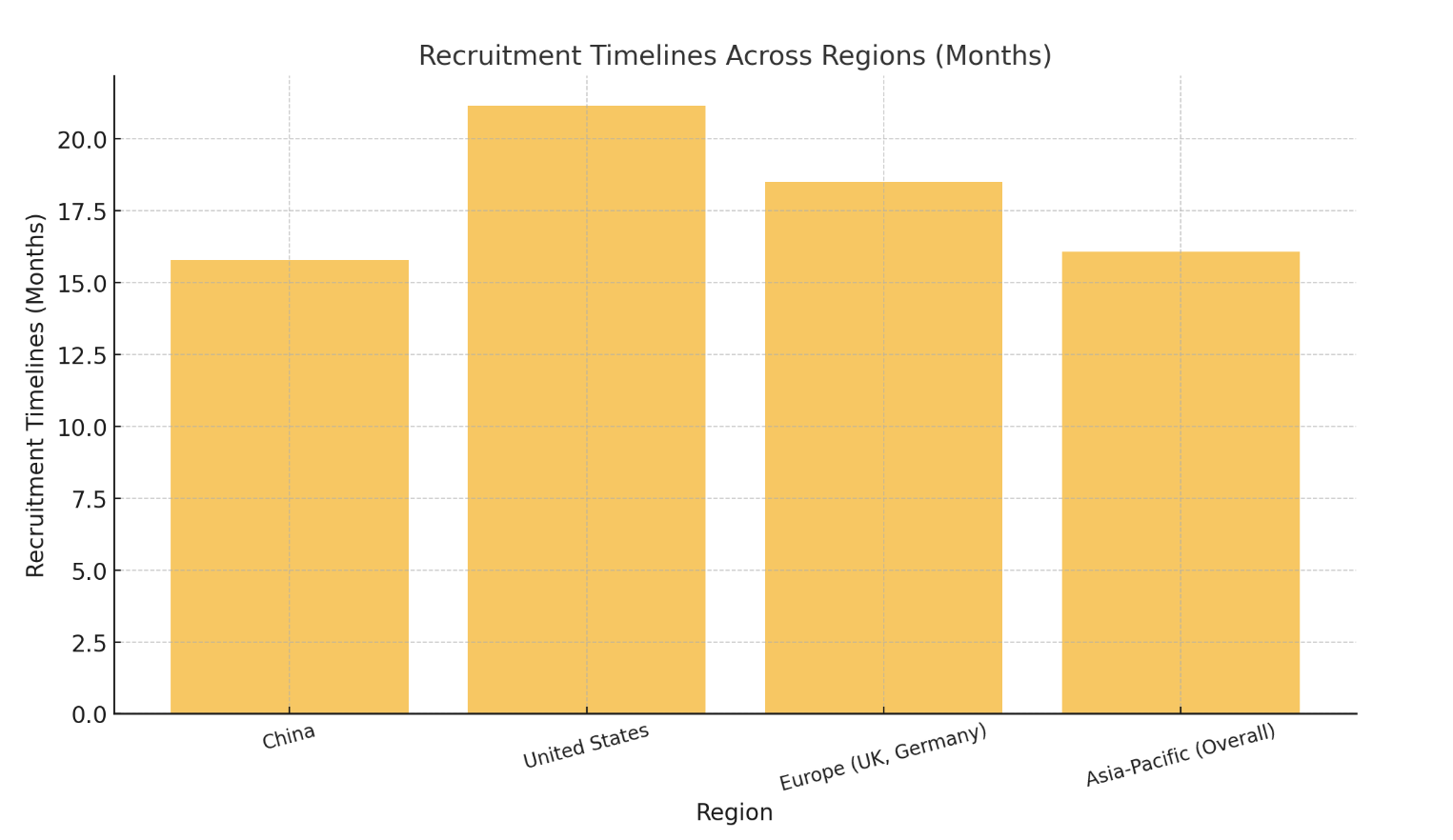
Asia-Pacific’s efficiency and cost-effectiveness make it an attractive hub for CGT development, enabling sponsors to accelerate timelines and optimize resources while expanding access to innovative therapies. This regional advantage is expected to drive continued growth and global partnerships in the years ahead.
Addressing Challenges and Seizing Opportunities
While the potential of CGT is vast, several challenges persist, including high costs, complex regulatory landscapes, and scalability issues. Transitioning from autologous to allogeneic therapies—where treatments are "off-the-shelf" rather than patient-specific—could address some of these hurdles. However, ensuring batch consistency and regulatory compliance remains critical.
Strategic Pathways Forward:
- Regulatory Adaptation: Agencies like the FDA and EMA are introducing expedited pathways such as PRIME and Breakthrough Therapy Designation to accelerate CGT approvals.
- Investment Surge: Despite a dip from the 2021 funding peak, venture capital continues to fuel innovation, focusing on start-ups and growth-stage companies.
- Global Collaboration: Partnerships between biotech innovators, academia, and CDMOs are key to advancing CGT accessibility and affordability worldwide.
The Path Ahead: A Transformative Horizon
By 2025, the FDA anticipates 10-20 CGT approvals annually, with over one million patients expected to benefit globally by 2034. As CGT transitions from experimental to mainstream medicine, stakeholders must balance innovation with operational pragmatism. Scaling up production, leveraging AI, and fostering global collaboration will be critical in unlocking the full potential of these therapies.
The CGT landscape exemplifies a dynamic confluence of science, technology, and strategy. As we navigate this era of medical breakthroughs, the collective efforts of researchers, developers, and regulators promise a future where previously untreatable diseases can be cured, offering hope to millions worldwide.






