By 2030, pharmaceutical companies are expected to lose over $236 billion in revenue due to the impending ‘patent cliff,’ as 190 drugs, including 69 blockbusters, lose exclusivity. This represents about 46 per cent of sales at risk for the top ten pharma companies over the next decade, according to a report by Deloitte.
This is currently what experts call ‘wave 2’ of patent expirations. The first wave (2010–2020) fueled the growth of the generics industry. Now, the second wave involves the expiration of key monoclonal antibody patents, such as Keytruda, presenting a massive multi-billion dollar opportunity for biosimilars.
To mitigate revenue loss from these blockbuster drugs, pharmaceutical companies are exploring various strategies, including partnerships, mergers and acquisitions, and patent thickets.
“Based on the current status of biosimilars in development, we will see more diversification of businesses (less pure players), more mergers, acquisitions and partnerships. For developers to continue to evaluate the investment required to launch future products in high-cost drug categories, there has to be a level of confidence that the market will support the lower cost biosimilars when they are approved years down the line," said Linda MacDonald, Executive Vice President and Commercial Division Lead at Samsung Bioepis.
Unlike generics, many biosimilars won’t be interchangeable or directly substitutable. Also, because biosimilars are more expensive to develop and manufacture, their producers won’t be able to reduce prices as significantly as generic drug makers do.
“My personal view is that the evidence so far suggests there will be a balance of both approaches and there will be fragmented strategies going forward. A lot will depend on the commercial, patent and technology situation around an individual biologic and - in the US at least - the formulary adoption status. In general, biosimilars present more challenges compared with standard chemical generics due to their manufacturing complexity, time and cost,” said Ian Haydock, Editor-In-Chief, Asia-Pacific, Insights at Citeline (formerly Pharma Intelligence).
Experts also believe that, although there will be a loss of revenue, the impact will be gradual and can be mitigated to some extent. Biosimilars uptake has been slower in the US than in Europe.
“A look at actual case studies including AbbVie’s mega-blockbuster Humira and others shows a variety of originator strategies, including price reductions, patent protection moves (including legal action), modified rebate practices, Pharmacy Benefits Manager (PBM) partnerships and patient assistance programmes. The development of new formulations and acquisition of novel successor products, either through in-house R&D or licensing/M&A activity, present other options. In Humira’s case, biosimilar erosion was carefully planned for by AbbVie and their forecasts turned out to be generally accurate (biosimilar penetration tends to be lower in chronic versus acute indications, for instance), showing that the patent cliff impact can be managed,” said Ian Haydock.
According to IQVIA, only 10 per cent of the molecules set to lose patent protection from 2025 to 2034 have biosimilars in development. And biosimilars take 7-10 years to develop and up to $500 million for development.
“The market has to be sustainable – tough, especially in the US. There is an enormous need for a thriving biosimilar market in the US as it is the best solution to high drug costs. The US is the single largest biologics market in the world for originators and the home to much of biotech investment – and that needs to continue,” said Linda.
Nonetheless, this shift is set to transform the pharma industry. The rise of biosimilars could also improve access to medicines, which, ultimately, should be the fundamental goal, ensuring that life-saving treatments are available to more people.
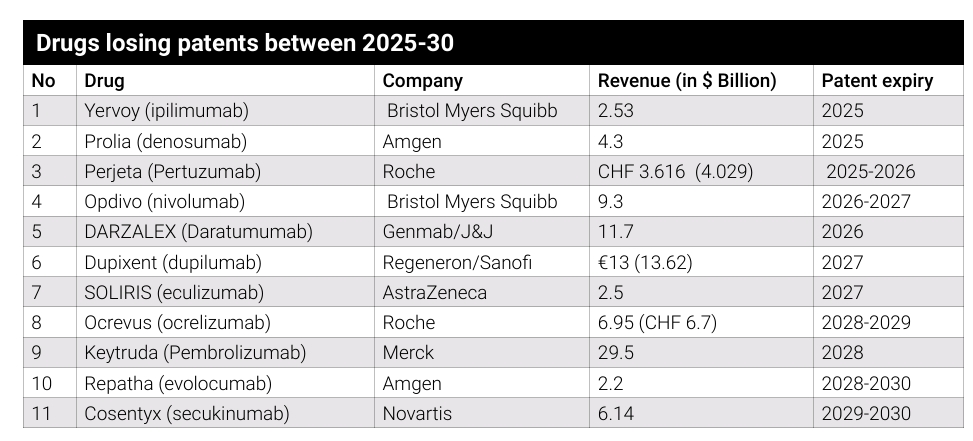
Key mABs’ patent expiring by 2030
Here, we highlight 11 monoclonal antibody patents (with revenue more than $1 billion) set to expire between 2025 and 2030, poised to impact some of the biggest players in the pharmaceutical industry.
Yervoy (ipilimumab)
Company: Bristol Myers Squibb (BMS)
2024 sales: $2.53 billion
Patent expiry: 2025
Ipilimumab, sold under the brand name Yervoy, is a monoclonal antibody medication that works to activate the immune system by targeting CTLA-4 and is used as an anticancer drug for lung cancer (NSCLC), melanoma, renal cell carcinoma, liver cancer, colorectal cancer, esophageal cancer, and pleural mesothelioma. Yervoy (ipilimumab) is one of BMS’ top selling cancer drugs and clocked $2.53 billion in 2024.
The exclusivity period is based on the composition of matter patent, which expires in 2025 and currently, no approved biosimilar is available, although few are in development. Ipilimumab biosimilar is under clinical development by Innovent Biologics and currently in phase II for Metastatic Biliary Tract Cancer. Another firm working on the biosimilar is Shanghai Henlius Biotech announced that the first subject was dosed for a phase 1 clinical trial of the company's independently developed ipilimumab biosimilar HLX13 (recombinant anti-CTLA-4 fully human monoclonal antibody injection) in China.
Prolia (denosumab)
Company: Amgen
2024 sales: $4.3 billion
Patent expiry: 2025
Prolia (denosumab) is a long-standing drug used primarily in the United States, Europe, and the Asia Pacific region. It contains the same active ingredient as XGEVA (denosumab), but it is approved for different indications, patient populations, doses, and frequencies of administration. Launched in the U.S. and Europe in 2010, Prolia is primarily used in the treatment of postmenopausal women with osteoporosis who are at high risk of fracture, either due to a history of osteoporotic fractures or multiple fracture risk factors.
In 2024, Prolia generated $4.3 billion in sales. However, the company anticipates a decline in sales starting in 2025 due to the upcoming launch of biosimilars, as the drug's exclusivity is set to expire this year.
The US FDA has already approved two biosimilars to Prolia. In 2024, the FDA approved AndozJubbonti (denosumab-bbdz) injection as an interchangeable biosimilar to US licensed Prolia. In February 2025, Samsung Bioepis received FDA approval for its biosimilar, OSPOMYV (denosumab-dssb, SB16), in a 60 mg pre-filled syringe. Additionally, Celltrion's denosumab biosimilar has been approved in the European Union. Teva's Prolia biosimilar candidate has also been accepted for review by both the US FDA and the European Medicines Agency (EMA), with approval expected later this year.
Perjeta (Pertuzumab)
Company: Roche
2024 sales: CHF 3.616 billion ($4.029 billion)
Patent expiry: 2025-2026
Pertuzumab (Perjeta) is a recombinant humanised monoclonal antibody that targets the dimerisation domain II of the HER2 receptor on the cell surface and is used for the treatment of breast cancer. It is one of the best selling cancer drugs and clocked CHF 3.616 billion.
The group’s basic primary patent expires in 2025 in the US and EU and the group currently anticipates biosimilar could be available in the market in 2026.
Several companies are developing biosimilars for Perjeta. Qilu Pharmaceutical Co., Ltd., based in Jinan, China, is developing QL1209, which is currently in phase 3 trials. Zydus Cadila and Dr. Reddy’s Laboratories have also entered into a licensing agreement to co-market Zydus' biosimilar of pertuzumab. In 2025, the FDA accepted a Biologics License Application (BLA) for a pertuzumab biosimilar developed by Shanghai Henlius Biotech, indicating progress in the competitive biosimilar market for this cancer treatment.
Opdivo (nivolumab)
Company: Bristol Myers Squibb
2024 sales: $9.3 billion
Patent expiry: 2026-2027
Opdivo (nivolumab) is a fully human monoclonal antibody that targets the PD-1 receptor on T and NKT cells, enhancing immune responses against cancer. Approved since 2014, Opdivo has received indications for several cancers, including melanoma, head and neck cancer, lung cancer, kidney cancer, and blood cancer. Additionally, the Opdivo + Yervoy regimen has been approved in multiple markets for melanoma treatment. Ongoing trials are exploring its potential in other tumor types and disease areas.
Opdivo is Bristol-Myers Squibb's top-selling cancer drug, with sales reaching $9.3 billion in 2024.
Bristol-Myers Squibb and Ono jointly hold the patent for nivolumab as a composition of matter, which is set to expire in the U.S. in 2027 and in the EU in 2026 (excluding potential patent term extensions). In Japan, the composition of matter patent for nivolumab will expire in 2031, including a granted patent term extension.
While Zydus Life Sciences' biosimilar nivolumab has been approved in India, several other companies are actively developing biosimilars. Sydney-based NeuClone Pharmaceuticals, in collaboration with Serum Institute of India, is advancing biosimilar candidates for nivolumab in the preclinical stage. Additionally, Swedish firm Xbrane Biopharma and Intas have partnered to jointly develop an Opdivo biosimilar, while China's Luye Pharma is also working on its version. Amgen’s Opdivo biosimilar is currently in phase 3 trials.
Darzalex (Daratumumab)
Company: Genmab/J&J
2024 sales: $11. 7 billion
Patent expiry: 2026
Daratumumab, sold under the brand name Darzalex, is an anti-cancer drug used to treat multiple myeloma. Originally developed by Genmab, the drug is now being jointly developed with Janssen Biotech, a subsidiary of Johnson & Johnson, which acquired worldwide commercialisation rights.
In 2024, Darzalex generated net sales of $11.67 billion, making it one of the best-selling anti-cancer drugs. The composition of matter patents for daratumumab in the U.S., Europe, and Japan are set to expire in March 2026.
Several companies are developing biosimilars to Darzalex. One such biosimilar, HLX 15, is being developed by Shanghai Henlius Biotech and licensed to Dr. Reddy’s Laboratories. HLX 15 is a humanised IgG1K monoclonal antibody indicated for the treatment of multiple myeloma.
On November 28, 2024, Celltrion announced the initiation of global phase 3 clinical trials for its biosimilar, CT-P44, which is based on daratumumab. This follows the submission of its global phase 3 clinical trial plan to the European Medicines Agency (EMA).
Additionally, Xdarzane, developed by Xbrane Biopharma, another biosimilar candidate to Darzalex, is currently in the preclinical development stage. Focused on creating a cost-effective production process, Xdarzane aims to demonstrate biochemical similarity to the original drug.
Dupixent (Dupilumab)
Company: Regeneron/Sanofi
2024 sales: €13 billion ($13.62 billion)
Patent expiry: 2027
Dupilumab, jointly developed by Regeneron and Sanofi and marketed under the brand name Dupixent, is a monoclonal antibody that blocks interleukin 4 (IL-4) and interleukin 13 (IL-13) receptor signaling (IL-4R, IL-13R). It is used to treat a variety of allergic diseases, including eczema (atopic dermatitis), eosinophilic or oral-corticosteroid-dependent asthma, chronic rhinosinusitis with nasal polyps, COPD with an eosinophilic phenotype, eosinophilic esophagitis (EOE), and prurigo nodularis.
Dupixent is one of the bestselling drugs, with 2024 sales reaching €13 billion ($13.62 billion) in the atopic dermatitis treatment market. Its patent (methods of treatment) starts to expire in 2027 and currently, Bio-Thera is the only company developing a biosimilar for Dupixent.
Soliris (eculizumab)
Company: AstraZeneca
2024 sales: $2.5 billion
Patent expiry: 2027
Eculizumab, sold under the brand name Soliris, is a recombinant humanised monoclonal antibody used to treat conditions such as paroxysmal nocturnal hemoglobinuria (PNH), atypical hemolytic uremic syndrome (aHUS), generalised myasthenia gravis, and neuromyelitis optica. It’s an important drug for AstraZeneca and cocked $2.5 billion in sales.
Although Soliris will lose its exclusivity by 2027, two biosimilars have already been approved by the US FDA in 2024. In May 2024, the FDA approved Bkemv (eculizumab-aeeb) as the first interchangeable biosimilar to Soliris. Developed by Amgen, Bkemv is approved for the treatment of PNH and aHUS, the same indications as Soliris.
In July 2024, the FDA also approved Epysqli (eculizumab-aagh), a biosimilar developed by Samsung Bioepis. Epysqli is approved for the treatment of PNH and aHUS, marking it as the second biosimilar to Soliris.
Ocrevus (ocrelizumab)
Company: Roche
Sales: $6.95 billion (CHF 6.7 billion)
Patent expiry: 2028-2029
Ocrevus (ocrelizumab) is a humanised anti-CD20 monoclonal antibody that targets the CD20 marker on B lymphocytes, a type of immune cell involved in multiple sclerosis (MS). It acts as an immunosuppressive drug and is the first and only therapy approved for both relapsing and primary progressive forms of MS. Ocrevus is administered biannually (every six months) and was first approved in 2017. Since then, it has become Roche’s top-selling drug, generating over CHF 6 billion ($6.95 billion) in sales last year and capturing around 22 per cent of the MS market.
According to GlobalData, Ocrevus’ patents are set to expire in Europe in 2028 and in the U.S. in 2029, paving the way for potential biosimilars to enter the market. Several companies are actively developing biosimilars for Ocrevus.
CinnaGen, the largest biotechnology company in Iran and the Middle East and North Africa region, has developed a follow-on biologic to ocrelizumab, called Xacrel. Xacrel was approved by the Iran FDA in 2021, and is currently available exclusively in Iran for treating both relapsing-remitting multiple sclerosis (RMS) and primary progressive multiple sclerosis (PPMS).
Amgen is also working on an ocrelizumab biosimilar, ABP 692, which is currently in phase 3 trials. The phase 3 study (NCT06700343) will assess the pharmacokinetic and pharmacodynamic similarity between ABP 692 and Ocrevus in patients with relapsing-remitting multiple sclerosis, with enrollment beginning in February 2025 in the US.
Celltrion is also developing an ocrelizumab biosimilar, CT-P53. In August 2023, the European Medicines Agency (EMA) granted partial approval for its Phase 3 Investigational New Drug (IND) application. The study (NCT05906992), which is currently recruiting participants in Poland, aims to further evaluate CT-P53.
Keytruda (pembrolizumab)
Company: Merck
2024 sales: $29.5 billion
Patent expiry: 2028
Keytruda (pembrolizumab) is an anti-PD-1 therapy that enhances the body’s immune system to detect and fight tumour cells. It is used to treat a variety of cancers, including melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer.
As one of Merck’s top-selling drugs, Keytruda has been a cornerstone of the company’s oncology portfolio. In 2024, Keytruda is projected to be the highest-ranked drug worldwide by sales, with Merck's global sales reaching $29.5 billion, a growth of 18 per cent from the previous year.
Keytruda is also one of the industry's most talked-about patent cliffs. The drug faces a significant patent expiration in 2028,which has spurred a wave of biosimilar development as companies aim for a share of this lucrative market. Major players, including Samsung, Amgen, Sandoz, and Celltrion, all are working on biosimilars.
In 2024, Samsung Bioepis initiated a phase III trial for its proposed Keytruda biosimilar, SB27. Formycon AG has also enrolled the first patient in the phase III "Lotus" trial, comparing the safety and efficacy of its biosimilar, FYB206, to Keytruda. Bio-Thera has started an integrated phase I/III trial for its biosimilar, BAT3306. Additionally, Celltrion received FDA approval for its phase 3 clinical trial plan for CT-P51, a biosimilar to Keytruda. Henlius has had its IND application for its pembrolizumab biosimilar approved by the NMPA in China.
Repatha (evolocumab)
Company: Amgen
2024 sales: $2.2 billion
Patent expiry: 2028-2030
Evolocumab, sold under the brand name Repatha, is a monoclonal antibody and immunotherapy medication used to treat hyperlipidemia. manufactured by Amgen, Repatha is a PCSK9 inhibitor that helps lower cholesterol levels.
In 2024, Repatha's sales increased by 36 per cent, reaching over $2.2 billion, making it one of Amgen's top-selling drugs.
The patent for Repatha is set to expire in the EU in 2028 and in the U.S. around 2029/30. CinnaGen is the only company developing a biosimilar to evolocumab, which is currently in phase III clinical trials for hyperlipidemia.
Cosentyx (secukinumab)
Company: Novartis
2024 sales: $6.14 billion
Patent expiry: 2029-2030
Cosentyx (secukinumab) is a monoclonal antibody classified as an interleukin (IL) inhibitor, specifically targeting the IL-17A protein. It is used to treat various autoimmune conditions, including plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, enthesitis-related arthritis, hidradenitis suppurativa (HS), and non-radiographic axial spondyloarthritis. By blocking IL-17A, Cosentyx helps reduce inflammation and is considered an immunosuppressant. Since its launch in 2015, Cosentyx has become a leading treatment in the psoriasis market and is one of Novartis’s best-selling drugs, with sales reaching $6.14 billion in 2024.
According to Novartis’ annual report, the patent for Cosentyx will expire in the US in 2029 and in the EU in 2030. Currently, no biosimilars for secukinumab (Cosentyx) have been approved, although some are in development. In 2024, Celltrion received FDA approval for its global Phase 3 clinical trial of CT-P55, a biosimilar to Cosentyx. Meanwhile, Bio-Thera is developing BAT2306, its own secukinumab biosimilar, which is also in phase 3 clinical trials.
Ayesha Siddiqui




